In their article, “A Novel Method Combining Vitreous Aspiration and Intravitreal AAV2/8 Injection Results in Retina-Wide Transduction in Adult Mice,” Da Costa et al use the Phoenix MICRON® III imaging platform to take stunning images demonstrating the success of their novel intravitreal injection technique. Gene therapy is a promising treatment option of genetic retinopathies—adeno-associated viruses (AAV) are already used to treat Leber’s congenital amaurosis. Effective delivery of the AAV without causing further retinal disruption is essential. Da Costa et al found that by aspirating a small amount of the vitreous before intravitreally injecting their GFP-linked AAV, the transduction efficiency was vastly superior to the traditional intravitreal injection technique.
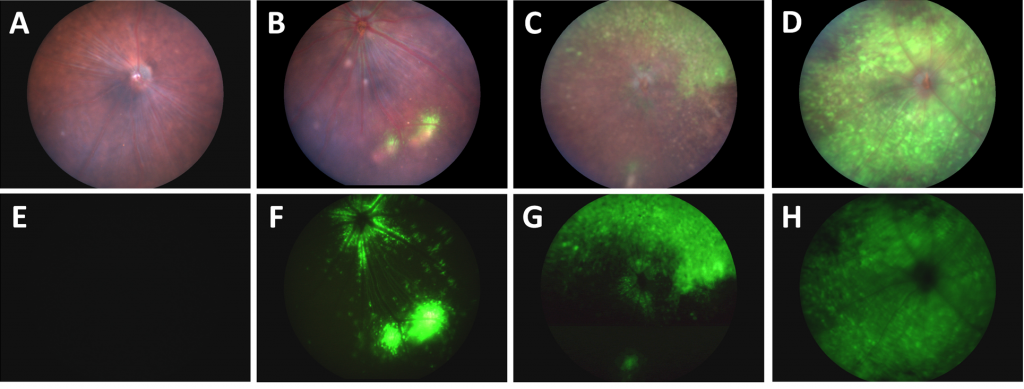
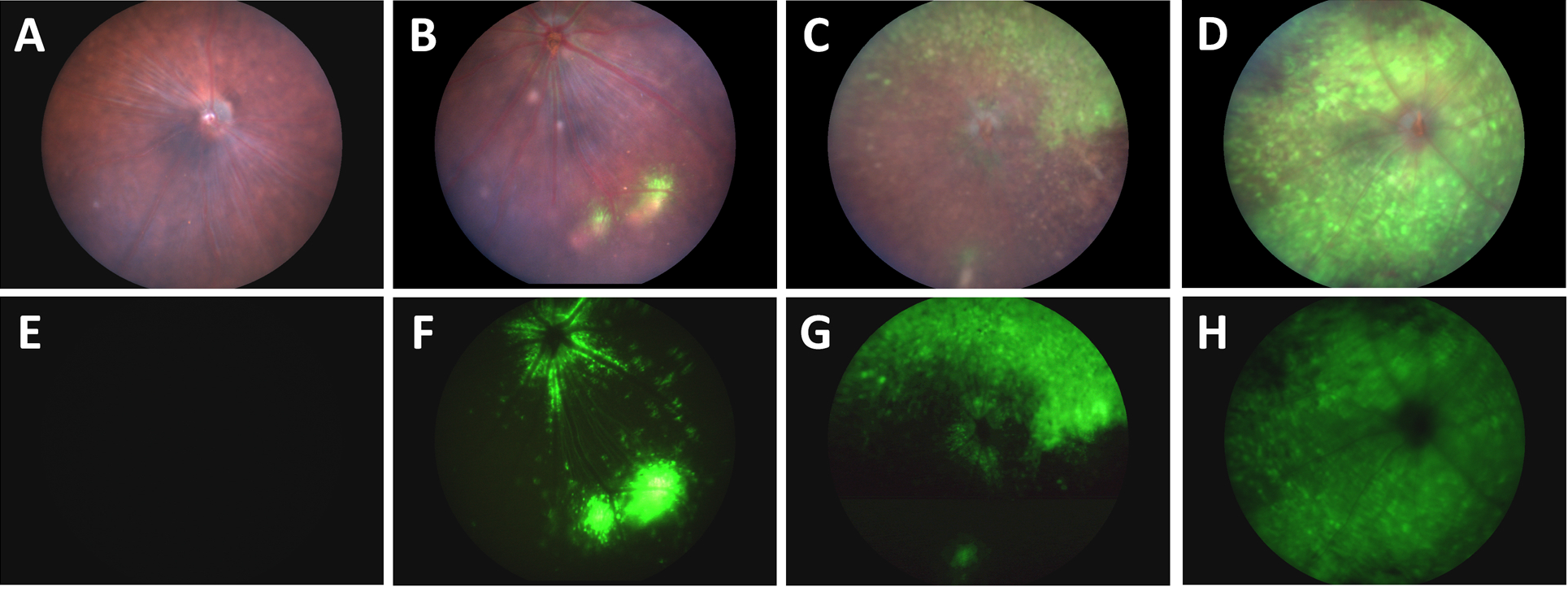
Using the Phoenix MICRON® III system to take fundus images in brightfield and with a GFP filter, Da Costa et al had four groups of mice with different injection techniques of a GFP-linked AAV2. The traditional intravitreal injection technique showed two to three spots of GFP-positive cells and some GFP around the optic nerve (Fig 1B, F). A second technique of making a small hole in the vitreous and applying pressure before injecting the AAV transduced a slightly larger area with GFP (Fig 1 C, G). The novel technique of aspirating some vitreous before injecting with the AAV showed a stunning expression of GFP all over the retina (Fig 1 D, H). As a control, mice injected with PBS using the novel technique did not have any GFP expression (Fig 1A, E).
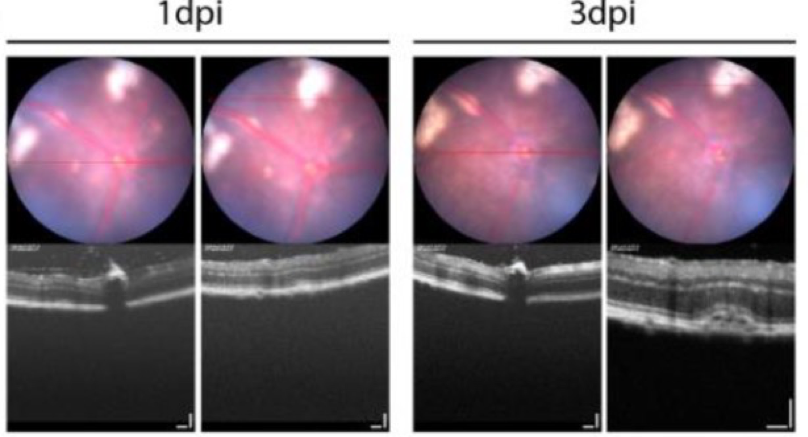
Da Costa et al used the Phoenix MICRON® OCT to confirm that the retina was not damaged by their novel injection technique—there was some retinal detachment in a few mice that resolved after a week (Fig 2). The new aspirate and then inject technique may work for three reasons, Da Costa et al theorize: 1. The vitreous, if not aspirated, may exert pressure that forces the viral suspension out of the eye, 2. The internal limiting membrane is disrupted by the aspiration, allowing the viral particles to reach the retina, and 3. Aspirating the vitreous reduces the number of native neutralizing antibodies.
This article is a wonderful demonstration of how the Phoenix MICRON® III can be used to easily assess retinal treatment or techniques over a long period of time. The GFP-positive fundus images in this article are stunning.
Citation: Da Costa, R., Röger, C., Segelken, J., Barben, M., Grimm, C., & Neidhardt, J. (2016). A Novel Method Combining Vitreous Aspiration and Intravitreal AAV2/8 Injection Results in Retina-Wide Transduction in Adult Mice. Investigative Ophthalmology & Visual Science, 57(13), 5326–5334.