In their 2019 paper, “Novel molecular mechanisms for Prph2‐associated pattern dystrophy,” Chakraborty et al use the Phoenix MICRON® IV retinal imaging platform to longitudinally study the effect of a very specific mutation affecting the Peripherin 2 protein. Peripherin 2 is a protein in rods and cones which, if mutated, can lead to retinitis pigmentosa, cone-rod dystrophy, and macular dystrophy. Despite being in cones and rods, peripherin 2 mutations can also lead to RPE and choroid defects. This in addition to the variability in the human phenotype even with the same mutation and the need for a certain level of the protein makes treatment extremely difficult. As such, there are currently no treatments for these diseases.
Many of the disease-causing mutations are in the key structural D2 region of peripherin 2. There are seven cysteines in the D2 region that affect bonding and structure. While a mutation in cysteine 213 leads to butterfly-shaped pattern dystrophy, a mutation in neighboring 214 leads to retinitis pigmentosa. There is a need to study each cysteine mutation thoroughly to lead to treatment.
Chakraborty et al use a mouse model with a cysteine 213 (C213Y) knock-in mutation. The mouse models are: Prph2C/+ (heteryozygous for mutation, similar to patients), Prph2C/C (homozygous for mutation), and the controls Prph2−/− (homozygous for the nonfunctional allele), Prph2+/− (heterozygous for the nonfunctional allele), and Prph2+/+(wild type).
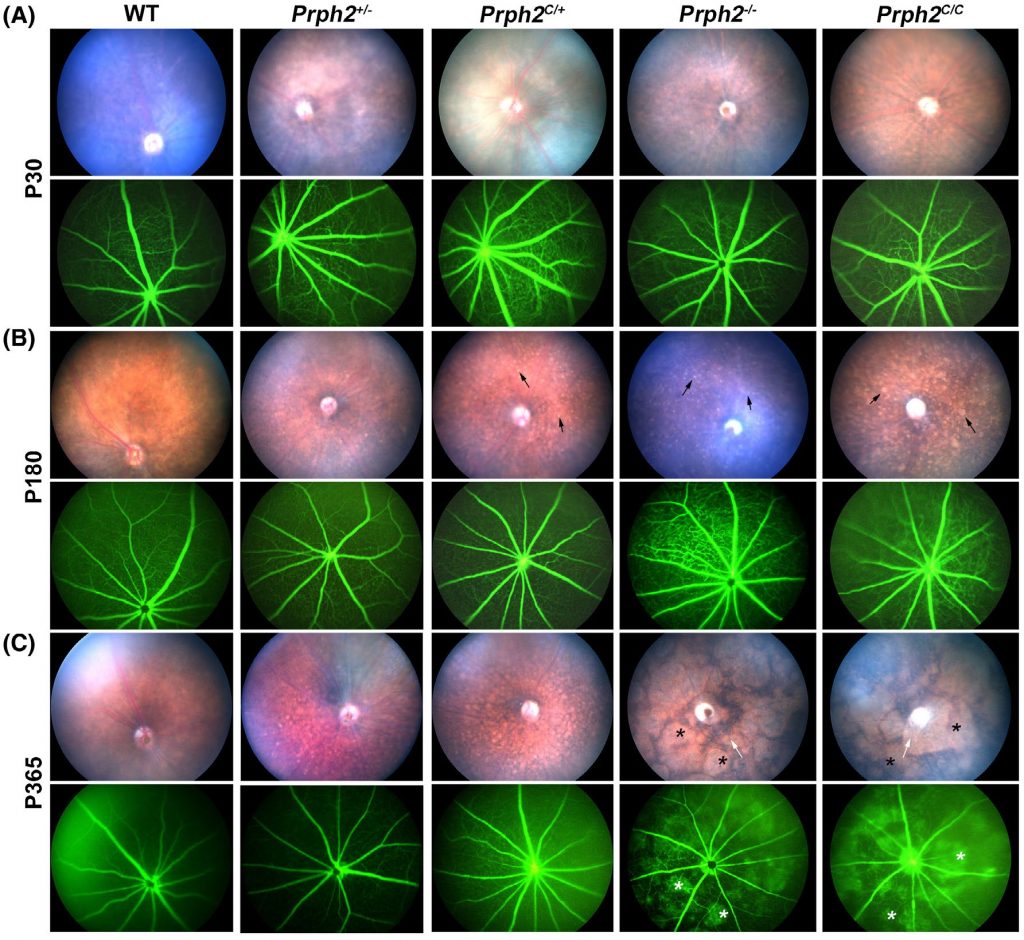
As seen in Figure 1, Chakraborty et al use the Phoenix MICRON® IV imaging camera to take beautiful fundus images of the different mutants over a long period of time—up to a year! This is an excellent demonstration of one of the ways the Phoenix MICRON can help research by allowing longitudinal studies instead of losing animals to histology at each time point.
The Phoenix MICRON® IV fundus images show a shift from normal fundus at P30 to major yellow flecking in the retinas of Prph2C/+, Prph2C/C, and mild yellow flecking in Prph2−/− retinas at P180. At P365, yellow flecking in Prph2C/+ and large splotchy areas in Prph2−/− and Prph2C/C are present. Fluorescein angiography, an imaging modality included with the basic Phoenix MICRON® IV model, is normal until P365 where Prph2−/− and Prph2C/C have leaky blood vessels (Fig 1).
The scotopic and photopic ERG had diminished wave amplitudes, meaning that a C213Y mutation affects rods and cones as opposed to a rod-dominant retinitis pigmentosa phenotype. Chakraborty et al attempted to rescue the phenotype with supplemental Peripherin 2. Prph2 overexpressing mice were crossed with the mutants but their Phoenix MICRON® IV fundus images were very variable. Some were the same as the C213Y mutants and some had reduced yellow flecking.
Chakraborty et al have elucidated the phenotypic effects of one cysteine mutation in a protein that causes variable diseases with very similar mutations. This may help lead to treatment for devastating sight-affecting diseases.
Chakraborty, D., Strayve, D. G., Makia, M. S., Conley, S. M., Kakahel, M., Al‐Ubaidi, M. R., & Naash, M. I. (2020). Novel molecular mechanisms for Prph2‐associated pattern dystrophy. The FASEB Journal, 34(1), 1211–1230.