In their paper, “Suppression of Choroidal Neovascularization by AAV-Based Dual-Acting Antiangiogenic Gene Therapy,” Askou et al develop an adeno-associated virus (AAV) treatment for age-related macular degeneration. Beautiful fluorescent fundoscopy performed with the Phoenix MICRON® validated the success of the subretinal AAV injection, while precise choroidal neovascularization (CNV) induced by Phoenix laser burns confirmed that the AAV does, indeed, decrease neovascularization.
Age-related macular degeneration leads to a crippling loss of vision through an overabundance of choroidal blood vessels. While there are limited treatments, more treatments and a cure are necessary. CNV induced by retinal laser burns in mice is used as the animal model of the disease; treatments that decrease laser burn-induced CNV are candidates for clinical treatments. The Phoenix laser is image guided and easy to use; a crisp fundus image and laser aiming beam allow for precise delivery of the laser burns and subsequent development of CNV.
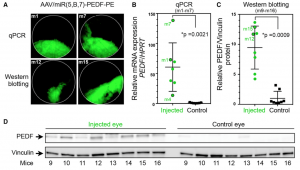
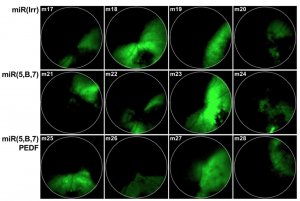
Askou et al developed two AAV; one contained microRNAs against vascular endothelial growth factor A (VEGFA), which mediates new blood vessel growth, and one also contained pigment endothelial-derived factor (PEDF) which decreases new blood vessel growth. Since the AAV also contained GFP, the Phoenix MICRON® fluorescent fundus images clearly showed where the virus was expressed in the retina (Figure 1). GFP expression was correlated with the anti-VEGFA micro RNAs and PEDF expression through qPCR and western blotting (Figure 2).
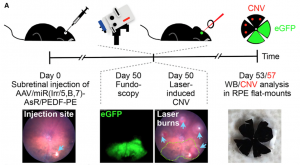
As Figure 3 shows, 50 days after AAV-injection, CNV was induced by using the Phoenix laser system by burning 50 micrometer spots in the retinal areas not affected by the AAV. Seven days later, retinas were excised and analyzed for CNV area and VEGFA levels. Askou et al found a significant decrease in CNV areas with both of the AAV, with a slightly larger decrease with the microRNA plus PEDF (45% for both vs 34% for one). VEGFA levels were decreased markedly by both AAV as well.
In the discussion, the researchers sum up their work thusly:
“In this study, we show that incorporation of the multigenic expression cassette into the AAV plasmid leads to the following: (1) expression of multiple proteins, including PEDF, in vitro following the transfection of relevant mammalian cells; (2) a high level of reporter gene expression in RPE cells following a single subretinal injection in mice; (3) co-expression of miRNA(5,B,7) and PEDF in eyes injected with multigenic AAV/miR(5,B,7)-PEDF-PE vectors; (4) reduced CNV formation in eyes treated with AAV vectors encoding therapeutic cassettes (AAV/miR(5,B,7)-AsR-PE and AAV/miR(5,B,7)-PEDFPE); and (5) an increased, albeit not statistically significant, efficacy due to combined expression of multiple antiangiogenic molecules (anti-VEGFA miRNAs and PEDF) from the same vector.”
The Phoenix MICRON® furthered their research by enabling points 2-3 with fundus imaging of the subretinal injections, and the Phoenix laser helped point 4 by easy induction of CNV through laser burns.
Askou, AL, Alsing S, Benckendorff, JNE, Holmgaard, A, Mikkelsen, JG, Aagaard, L, Bek, T, Corydon, TJ. (2019). Suppression of Choroidal Neovascularization by AAV-Based Dual-Acting Antiangiogenic Gene Therapy. Mol Ther Nucleic Acids. 16:38-50. doi: 10.1016/j.omtn.2019.01.012