Retinal vein occlusion is the second most common retinal vascular disorder leading to loss of vision in developed countries (after diabetic retinopathy). A lack of treatment options is partially caused by a lack of effective animal models; most rodent models do not show the essential symptom of cystoid edema and often heal quickly. In their article, “A pharmacological approach in newly established retinal vein occlusion model,” Fuma et al use the Phoenix MICRON® laser to develop a robust mouse model by systematically occluding retinal veins in ddY mice and then examining the retinal layers over time with the Phoenix MICRON® OCT.
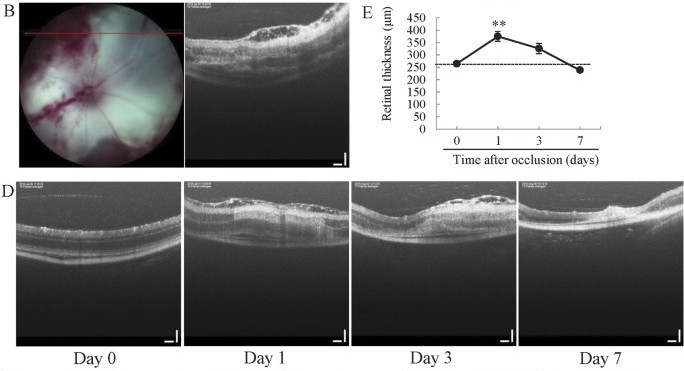
Fuma et al performed retinal vein occlusion using the Phoenix MICRON® laser on BALB/C, C57BL/6, and ddY mouse strains but found that only the ddY mice showed both cystoid edema and hemorrhage. The ddY mouse strain was thus determined to be the best model. The Phoenix MICRON® laser was used at very low power and high duration pulses with 10-15 laser burns per vein on three veins per eye (Fig 1B). This contrasts with most uses of the Phoenix MICRON® laser using high power and low duration to create choroidal neovascularization. Fuma et al examined the retinal layers using the Phoenix MICRON® OCT at 1, 3, 7, 14, 30, 90, and 180 days after laser (Fig 1D), demonstrating the power of longitudinal measurements made easy with the Phoenix MICRON® system.
The cystoid edema lasted for three days while the retinal thickness increased one day after laser burning but gradually decreased until returning to normal at day seven (Fig 1D, E). The retinal thickness was measured with the Insight 2D software included with the Phoenix MICRON® OCT which semi-automatically segments retinal layers and provides thickness measurements in micrometers.
After developing a robust mouse model of retinal vein occlusion, Fuma et al treated the mice with anti-VEGF drugs one day or seven days after laser occlusion. Perhaps explaining the variability in outcomes in human patients, the day one administration helped by decreasing the nonperfused retinal area. In contrast, the day seven administration caused harm by increasing the nonperfused retinal area. This highlights the importance of robust rodent models and timing the treatment of retinal vein occlusion carefully. This well written paper offers an excellent path for studying retinal vein occlusion and developing new, more effective treatments.
Citation: Fuma, S., Nishinaka, A., Inoue, Y., Tsuruma, K., Shimazawa, M., Kondo, M., & Hara, H. (2017). A pharmacological approach in newly established retinal vein occlusion model. Scientific Reports, 7, 43509.