Progress in diagnosing and treating Alzheimer’s disease (AD) has been accelerating over the last few years. Until now, AD has been difficult to diagnose. The disease can be present years before distinguishable symptoms manifest. Non-invasive diagnostic tests have been lacking, and diagnosis often relies on memory, cognitive, and behavioral tests. In 2011, Koronyo-Hamaoui M, Koronyo Y, Ljubimov AV, et al. at Cedars-Sinai Medical Center and the University of Southern California, Los Angeles, using the Phoenix MICRON® imaging platform, first demonstrated that the mouse retina suffers pathological changes that reflect the progress of AD. Now, new breakthrough research from Ferreira H, Serranho P, Guimarães, et al. at the University of Coimbra in Portugal is taking AD diagnosis into the age of artificial intelligence. This group of researchers leveraged the information captured by the MICRON Image-Guided OCT2 system from adult mouse retinal images to train an Artificial Intelligence (AI) model for diagnosing AD with high accuracy. Combining this breakthrough with the recent FDA approval of Leqembi, developed by Eisai in Japan and Biogen in Worcester, Massachusetts4, could lead to both non-invasive diagnosis and effective treatment options for this elusive disease.
What do we know about AD and its diagnosis?
Alzheimer´s disease (AD) is an age-related neurodegenerative condition that affects parts of the brain that control thought, memory, and language, with memory loss being the most common early clinical symptom. A hallmark of AD is the accumulation of the β-amyloid precursor protein in extracellular aggregates, called Aβ-plaques, that disrupt neuronal activities affecting communication in the brain. But AD diagnosis is not straightforward.
To diagnose AD, physicians perform memory, thinking, and other behavioral tests because biological non-invasive techniques are lacking. Indeed, in some cases, a definite diagnosis is made after death by looking for Aβ-plaques in the brain. Therefore, AD can remain undiagnosed for years since the manifestation of clinical symptoms could occur a decade after the beginning of the pathological changes in the brain. Thus, there is an urgent need for early and non-invasive detection of AD that could facilitate earlier treatment to prevent or delay the disease’s progression.
What is the connection between the eye and the brain in AD?
In a recent study, scientists discuss how mapping changes in the retina can alert to an earlier AD diagnosis, which could lead to an easy and practical way of finding signs of AD in patients. In this study, Koronyo et. al.1 characterized the pathological changes that occur in the retina of human subjects. Analyzing post-mortem retinal samples, the researchers found that the accumulation of Aβ-plaques was correlated with the severity of the disease and the upregulation of genes related to inflammation, neurodegeneration, and energy production inhibition.
But this journey started over a decade ago, when scientists from the Cedars-Sinai Medical Center and the University of Southern California, Los Angeles, using the Phoenix MICRON imaging platform, first demonstrated2 that the mouse retina suffers pathological changes that reflect the progress of AD. The discovery fueled many subsequent studies into the eye-brain relationship in both the clinical and pre-clinical environments.
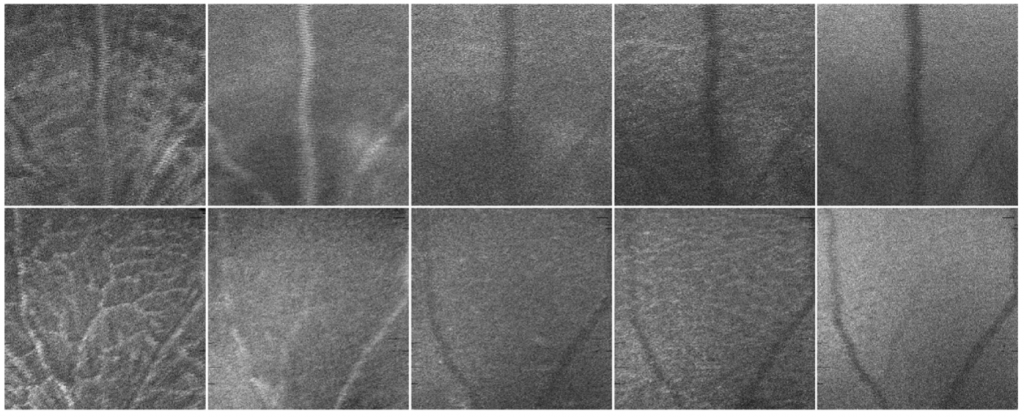
From that very first study, fast forward twelve years, and mounting evidence, another research group from the University of Coimbra in Portugal leveraged the information captured by the MICRON Image-Guided OCT2 system from adult mouse retinal images to train an Artificial Intelligence (AI) model for diagnosing AD with high accuracy. In this study, Ferreira et. al.3 investigated the way of using an AI system inspired by the brain function, called neural networks (NN), to detect AD signs in the retina of mice. The team created a system that uses computational models to mimic the function of neurons and discriminate signals into different groups or categories. By using optical coherence tomography (OCT) images taken with our MICRON IV OCT system, the scientists trained the NN to classify retinal images either as control (from healthy mice) or AD samples (from an AD mouse model). When testing the discrimination ability of the NN the team realized it was able to classify the retinal images into the control or AD group with an accuracy between 80 and 90%. This capacity of high discrimination supports the potential to use non-invasive retinal images in the early diagnosis and progression monitoring of AD and marks an important breakthrough in the translation of research in the lab that can be used in the clinic.
The Past and Future of AD Diagnosis
Now, 12 years after the first laboratory study described the correlation between the eye and the brain showing signs of AD, scientists are harnessing research discoveries and new pharmaceuticals to implement in the clinic in the near future.
And all in all, the work of many pre-clinical and clinical research groups brings us closer to the current trends of using AI and neural networks to classify healthy and AD retinas using non-invasive retinal imaging, reinforcing the opportunity to diagnose AD in humans before the first neurological symptoms appear.
References:
1- Koronyo Y, Rentsendorj A, Mirzaei N, et al. “Retinal pathological features and proteome signatures of Alzheimer’s disease.” Acta Neuropathol. 2023;145(4):409-438. doi:10.1007/s00401-023-02548-2
2- Koronyo-Hamaoui M, Koronyo Y, Ljubimov AV, et al. “Identification of amyloid plaques in retinas from Alzheimer’s patients and noninvasive in vivo optical imaging of retinal plaques in a mouse model.” Neuroimage. 2011;54 Suppl 1:S204-S217. doi:10.1016/j.neuroimage.2010.06.0202-
3- Ferreira H, Serranho P, Guimarães P, et al. “Stage-independent biomarkers for Alzheimer’s disease from the living retina: an animal study.” Sci Rep. 2022;12(1):13667. Published 2022 Aug 11. doi:10.1038/s41598-022-18113-y
4- Leqembi FDA announcement
Image credit:
Ferreira H, Serranho P, Guimarães P, et al. “Stage-independent biomarkers for Alzheimer’s disease from the living retina: an animal study.” Sci Rep. 2022;12(1):13667. Published 2022 Aug 11. doi:10.1038/s41598-022-18113-y