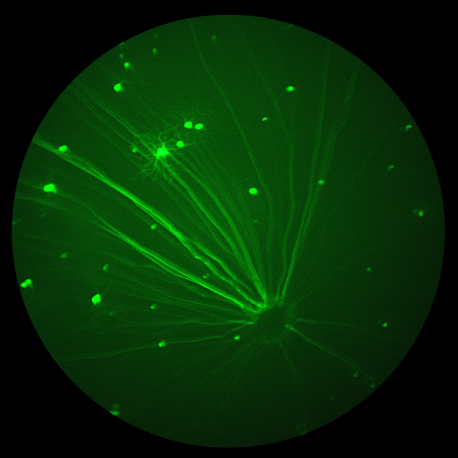
A team of researchers at the Indian Institutes of Technology have published three detailed articles examining how to improve adeno-associated viruses (AAV). Maurya, S, Mary, B, Jayandharan, GR et al -approach the improvement of the viruses in a stunningly detailed gene-to-cell-to-whole-mouse model, narrowing down a multitude of options and producing impressive fluorescent fundus images and Ganzfeld ERG traces with the Phoenix MICRON® and Phoenix Ganzfeld ERG.
Adeno-associated viruses are essential therapeutic tools that have already shown promising results in improving genetic conditions such as hemophilia and Leber’s congenital amaurosis. However, since they are viruses, they can set off negative immune responses which results in treatment at sub-optimal levels. The team of researchers sought to improve the efficacy of adeno-associated viruses paired with green fluorescent protein (GFP) and/or RPE65. GFP allowed measurement of the increase in fluorescent protein expression with Phoenix MICRON® fluorescent imaging. RPE65 treated visually impaired mice which whose vision rescue was measured with the Phoenix Ganzfeld ERG.
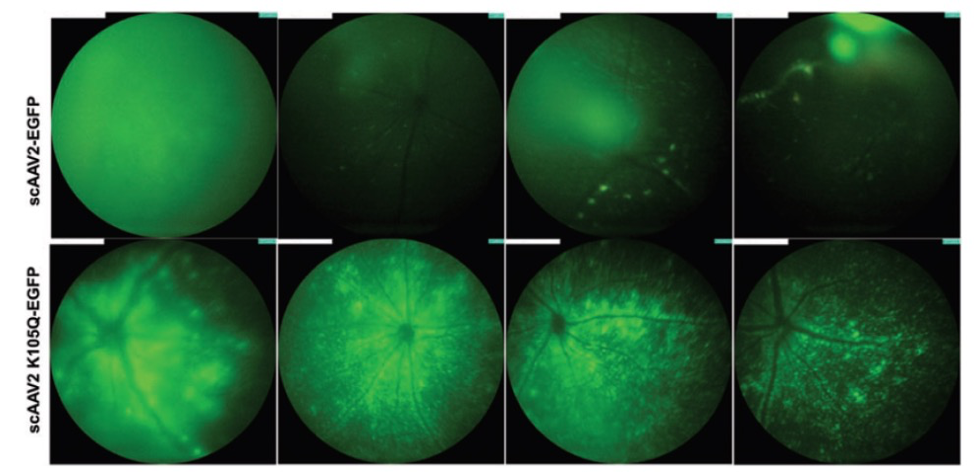
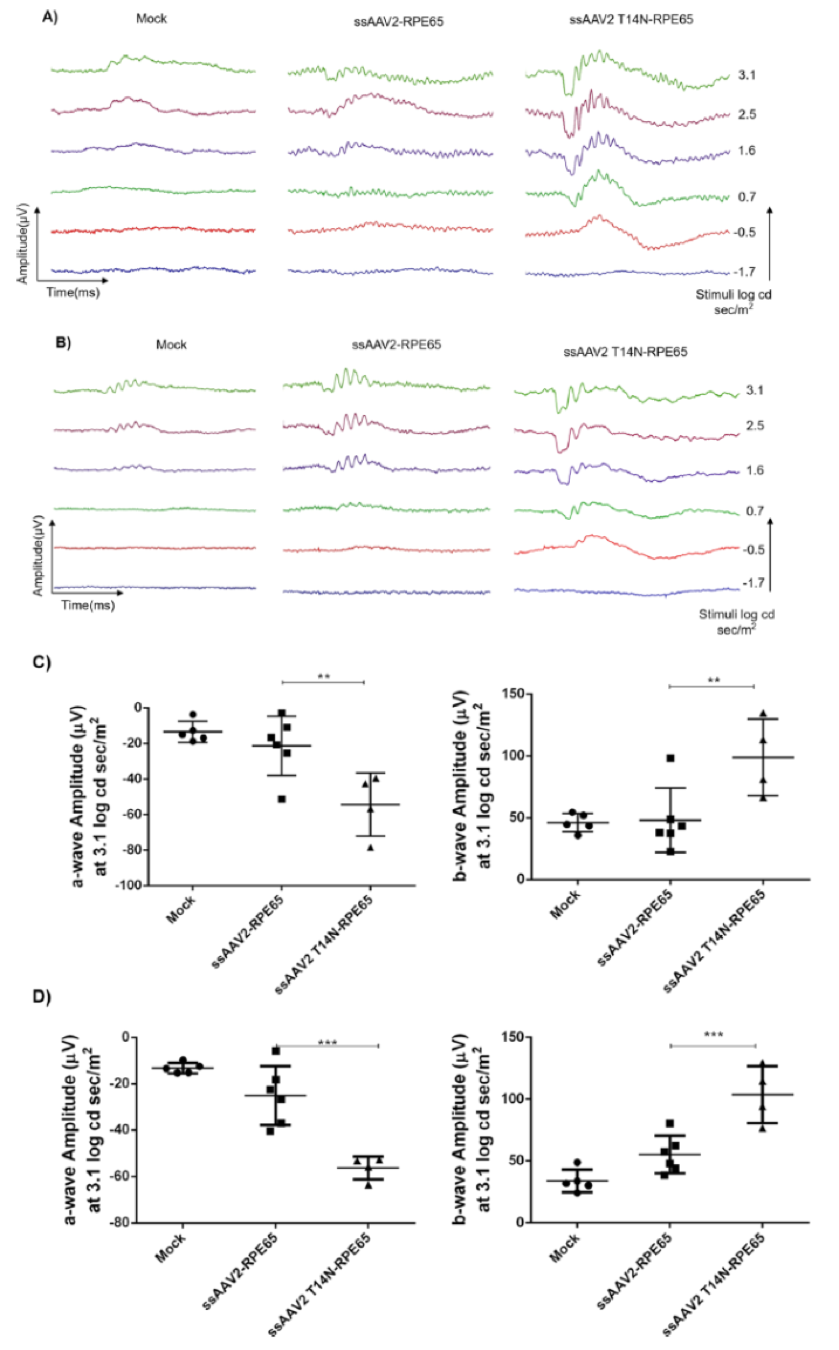
In “Rational Engineering and Preclinical Evaluation of Neddylation and SUMOylation Site Modified Adeno-Associated Virus Vectors in Murine Models of Hemophilia B and Leber Congenital Amaurosis,” Maurya, S, Mary, B, and Jayandharan, GR increased the virus efficacy by altering ubiquitin-like modifiers. When linked with GFP, this improved virus showed a 1.6-fold increase in GFP expression as examined with the Phoenix MICRON® fluorescent imaging (Fig 1). When the improved virus was paired with RPE65 and injected sub-retinally into a mouse model for Leber’s congenital amaurosis (rd12 mice), a genetic disorder resulting in severe vision loss or blindness, the mice demonstrated improved visual function as measured by Ganzfeld ERG compared to mice injected with the original virus paired with RPE65 (Fig 2).
In “Molecular Engineering of Adeno-Associated Virus Capsid Improves Its Therapeutic Gene Transfer in Murine Models of Hemophilia and Retinal Degeneration,” Mary, B, Maurya, S et al altered glycosylation sites to improve gene expression. Phoenix MICRON® fluorescent images revealed a 2-4 fold increase in GFP in mice injected with the improved virus paired with GFP (Fig 3). The Phoenix Ganzfeld ERG revealed a higher rate of photoreceptor response in a mouse model of Leber’s congenital amaurosis injected with the improved virus paired with RPE65 than those injected with the original virus (Fig 4).
Adeno-associated viruses go through post-translational modifications that are poorly understood and may affect the efficacy of the vector. In “Post-translational modifications in capsid proteins of recombinant adeno-associated virus (AAV) 1-rh10 serotypes,” Mary, B, Maurya, S et al examined several different types of post-translational modifications and test the effects. One post-translational modification lead to lower expression of GFP as seen with the Phoenix MICRON® (Fig 5).
Article citations:
Maurya, S, Mary, B, Jayandharan, GR. (2019). Rational Engineering and Preclinical Evaluation of Neddylation and SUMOylation Site Modified Adeno-Associated Virus Vectors in Murine Models of Hemophilia B and Leber Congenital Amaurosis. Hum Gene Ther. 30(12):1461-1476.
Mary, B, Maurya, S, Kumar, M, Bammidi, S, Kumar, V, Jayandharan, GR. (2019). Molecular Engineering of Adeno-Associated Virus Capsid Improves Its Therapeutic Gene Transfer in Murine Models of Hemophilia and Retinal Degeneration. Mol Pharm. 16(11):4738-4750.
Mary, B, Maurya, S, Arumugam, S, Kumar V, Jayandharan, GR. (2019). Post-translational modifications in capsid proteins of recombinant adeno-associated virus (AAV) 1-rh10 serotypes. FEBS J. 286(24):4964-4981.
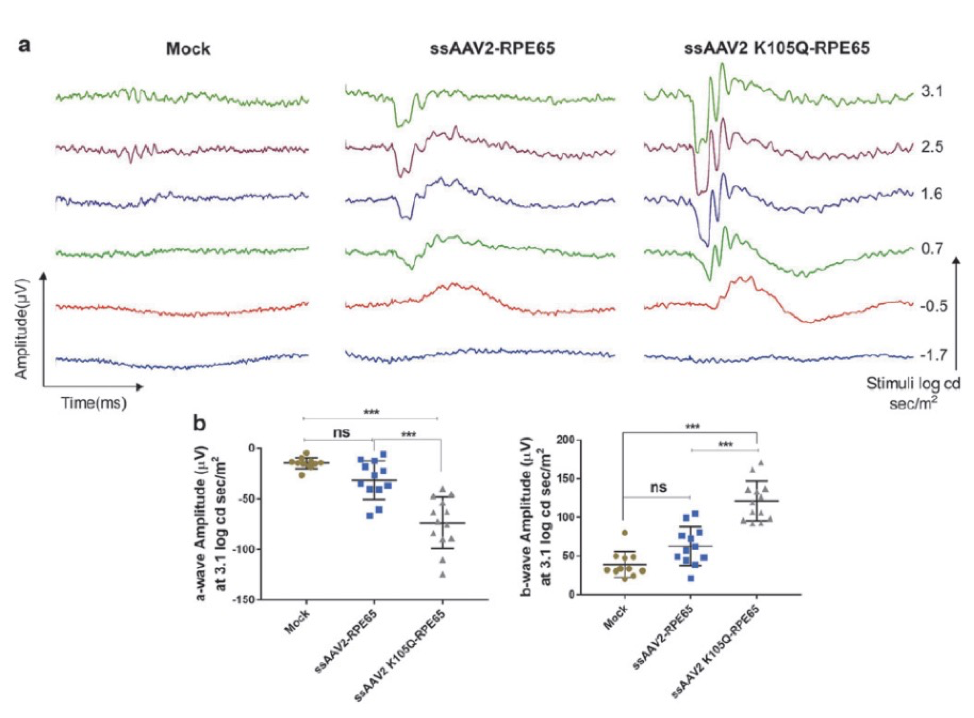
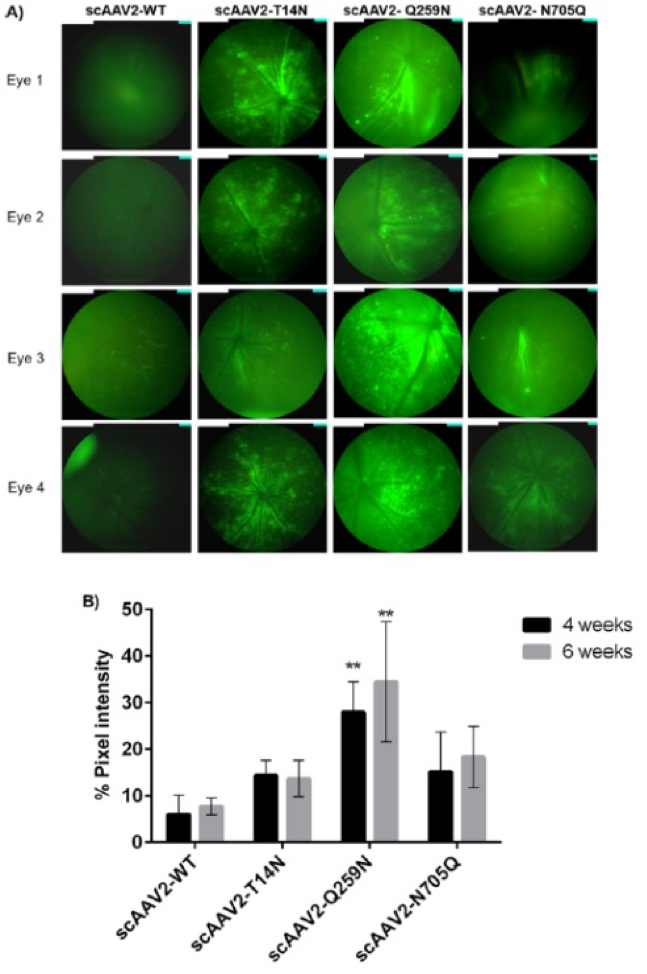
mice. In “Molecular Engineering of Adeno-Associated Virus Capsid Improves Its Therapeutic Gene Transfer in Murine Models of Hemophilia and Retinal Degeneration.”
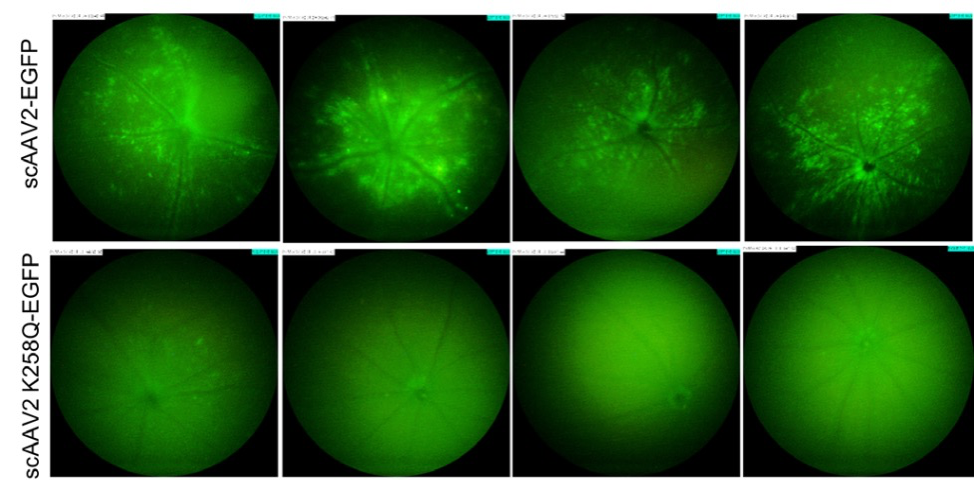
imaging of murine eyes, 2 weeks after intravitreal gene transfer with vectors. The standard fundus imaging in the Micron IV system (Phoenix Research Laboratories, Pleasanton, CA, USA) was performed with a mouse objective and a field of view at 50° (1.8-mm diameter). The images demonstrate GFP expression from representative eyes (n = 4) from a total of six eyes administered for each group. All the
images were captured at a uniform exposure setting for both the groups [Intensity at maximum, gain at 18 db (decimal gain), frame rate at 2 fps (frames per second)]. In “Post‐translational modifications in capsid proteins of recombinant adenoassociated virus (AAV) 1‐rh10 serotypes.”