The most common cause of blindness in developed societies, age-related macular degeneration (AMD) robs the victim of the crucially important focused macular vision. While still devastating, wet AMD has a robust mouse model and the treatment option of anti-vascular endothelial growth factor (VEGF) subretinal injections. The Phoenix MICRON® laser allows precise delivery of 532 nm energy on the rodent retina to break Bruch’s membrane and induce choroidal neovascularization (CNV) and is extensively used in wet AMD research. Dry AMD, however, does not have a robust mouse model or treatment. Ibbett et al, in their paper, “A lasered mouse model of retinal degeneration displays progressive outer retinal pathology providing insights into early geographic atrophy,” ,” use the Phoenix MICRON® laser injector with their own 810 laser to develop an elegant mouse model that mimics the human dry AMD symptoms and will be crucial in the search for treatment.
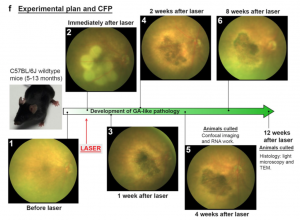
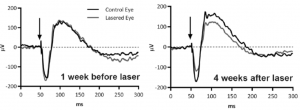
The Phoenix MICRON® provided clear color images of the longitudinal development of the laser burns from distinct spots to a merged geographic atrophy area very similar to the human dry AMD fundus (Figure 1). Using the contralateral eye as a control, the Phoenix MICRON® focal ERG revealed that the lasered eyes had decreased function as shown by decreased amplitude a- and b-waves (Figure 2). OCT scans, histology, and electron microscopy all confirmed similarity to the human condition.
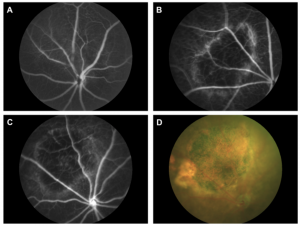
Ibbet et al used low power, high duration burns at 32 mW for 60 seconds to create the geographic atrophy. The laser burns morphed from distinct puncta to a merged atrophic area. Importantly, there were no signs of the CNV that is the hallmark of wet AMD (Figure 3). Several retinal layers degenerated to the point of being undetectable: the outer nuclear layer and inner/outer segment layers were not visible in OCT or histology, and the outer plexiform layer and retinal pigment epithelium and Bruch’s membrane morphology was altered. Histology revealed that the photoreceptors were destroyed in the lasered areas and that there was a distinct gradient from damaged to undamaged retinal areas. In the live fundus imaging, OCT, ERG, histology, and electron microscopy, the laser mouse model was remarkably similar to the human dry AMD condition.
Prior to this research, dry AMD rodent models included isolated features of dry AMD from genetically modified animals who had to be aged a very long time. Expensive and poor representations of the whole disease, these models were not satisfactory for a comprehensive study of dry AMD. In their thorough, molecule-to-mouse paper, Ibbett et al demonstrate an acute, consistent, and inducible rodent model of dry AMD, which is desperately needed for studying the pathology and potential treatments for the debilitating disease.