Dr. Rafael Ufret-Vincenty’s lab at University of Texas Southwestern Medical Center has developed a novel model for light damage using the Micron IV rodent retinal imaging camera. This quick and consistent light damage model leads to fundus abnormalities and retinal thinning as measured by the Micron image-guided OCT and semi-automated layer analysis tool, Insight. In two elegant articles, the researchers provided proof of concept in pigmented mice, which are a better model for human eye light damage than overly sensitive albino mice, which demonstrated bleached fundus and outer retinal layer thinning. In the second article, mutant mice lacking three oxidative stress-response proteins displayed even more damage than the wild type mice, validating the light damage model in oxidative stress studies. Using the new tool Insight 3D, researchers showed that the mutant mice were more sensitive to light damage, with more loss of outer retinal layer volume than the wild type mice. Dr. Rafael Ufret-Vincenty’s lab has developed a novel, consistent, and validated model of light damage using the Micron IV that can be used to study a wide range of retinal degeneration and light damage in a method that is more relevant to the human condition.
Light-induced retinal degeneration is used to study oxidative stress and inflammation as well as diseases such as advanced macular degeneration and some retinal dystrophies. While most light-induced retinal degeneration studies are done in light-sensitive albino mice and rats, Dr. Ufret-Vincenty’s lab examined pigmented, wild type C57Bl/6J mice which provide a more realistic model for humans, who can withstand a relatively high level of light before incurring damage. This novel model showed lead to retinal damage and thinning in both wild type mice and mice deficient in oxidative stress-response proteins.
In the first article, “Fundus Camera-Delivered Light-Induced Retinal Degeneration in Mice With the RPE65 Leu450Met Variant is Associated With Oxidative Stress and Apoptosis,” Zhong et al developed two models of fundus camera-induced light damage, one with fluorescein injection and one without. In the fluorescein models, the damage was greater as the concentration of fluorescein and the time between injection and light exposure increased. Though the light-only model also produced retinal damage, the light intensity and exposure time needed was much higher and the damage was less severe.
In the fluorescein model, light damage manifested as retinal pigment epithelium mottling, atrophy, and disruption, and thinning of the outer retinal layers as seen in the Micron IV OCT (Fig 1). In the light-only model, the fundus showed pigment changes and the OCT showed hyper-reflectivity of the outer retinal layers (Fig 2). As these are consistent with previous light damage studies using albino rodents, this fundus camera delivered light-induced retinal degeneration model is elegantly simple and more realistic to human eye conditions.
In the second article, “Increased susceptibility to fundus camera-delivered light-induced retinal degeneration in mice deficient in oxidative stress response proteins,” Ding et al then applied this light damage model to mice deficient in oxidative stress-response proteins and found the damage was more extensive in the deficient mice. They used both the light-only and fluorescein-assisted light damage with the Micron IV xenon lamp and measured damage via fundus images and the Micron OCT. The Micron OCT takes volume images of the retina which are analyzed with Insight 3D, providing the volume of retinal layers. The deficient mice had both more damaged fundus (Fig 3), they also had significantly reduced outer layer retinal volumes (Fig 4).
Using the Micron IV retinal imaging camera, OCT, and retinal layer analysis tool Insight 3D, Dr. Rafael Ufret-Vincenty’s lab has developed a novel, consistent, and validated model of light damage using the Micron IV that can be used to study a wide range of retinal degeneration and light damage in a method that is more relevant to the human condition.
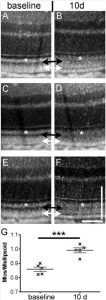
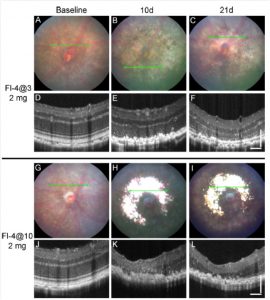
Figure 1. Fundus photos and OCT images from three time points in eyes exposed to the high dose of fluorescein and to the two light exposure protocols. Representative B6J eye at baseline (A, D), 10 days after (B, E), and 21 days after (C, F) exposure to 4 minutes of 54 K lux starting 3 minutes after the intraperitoneal injection of 2 mg fluorescein (Fl-4@3). Representative B6J eye at baseline (G, J), 10 days after (H, K), and 21 days after (I, L) exposure to 4 minutes of 54 K lux starting 10 minutes after the intraperitoneal injection of 2 mg fluorescein (Fl-4@10).
Figure 2. Optical coherence tomography images and reflectivity analysis before and after the ‘‘Light-only’’ FCD-LIRD protocol. Optical coherence tomography images of three different C57BL/6J eyes are shown before treatment (A, C, E) and 10 days after treatment (B, D, F) with 125 K lux for 30 minutes. The black arrows show increased reflectivity of the photoreceptor outer segments after light exposure. The white arrows show a new hyporeflective band just above the RPE after light exposure. There also is decreased reflectivity of the interdigitation zone (area between the black and white arrows) after light exposure. (G) Reflectivity analysis of the outer segments (black arrows) compared to the ellipsoid zone (white asterisks) in five C57BL/6J eyes before and after light exposure.
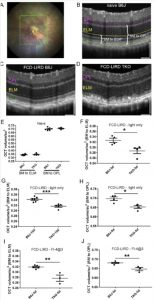
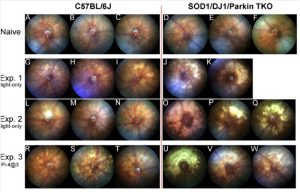
Figure 3. Mice simultaneously deficient in SOD1, DJ-1 and Parkin (triple KO, TKO) have a marked increase in retinal injury after exposure to the fundus camera-delivered light induced retinal degeneration (FCD-LIRD) models, when compared to C57BL/6 J mice. Fundus photos were obtained at baseline (AeF) or 5 days after light exposure (GeW). In experiment 1 (GeK) and experiment 2 (LeQ) eyes were exposed to the light-only protocol (30 min of 100 Klux of white light). In experiment 3 (ReW), eyes were exposed to a fluorescein-assisted protocol (2 mg of fluorescein i.p. followed 3 min later by a 4 min exposure to 54 Klux of white light).
Figure 4. Fundus camera-delivered light induced retinal degeneration (FCD-LIRD) leads to a significant decrease in outer retinal OCT volume in SOD1/DJ1/Parkin-deficient mice (TKO mice) compared to C57BL/6 J (B6J). The OCT volume was measured in a 5 disc diameter by 5 disc diameter square centered on the optic nerve head (A). Representative OCT images at baseline (B) and after FCD-LIRD (C, D) are shown. Measurements were made either from the external limiting membrane to the outer aspect of the RPE hyper-reflective band (“BM to ELM”; see E, F, G, I), or from the top of the outer plexiform layer to the bottom of the RPE hyper-reflective band (“BM to OPL”; see E, H, J). A statistically significant decrease in outer retinal volume was seen both after the “light-only” FCD-LIRD protocol (30 min of 100 Klux of white light; see F, G, H), and after the “fluorescein-assisted” FCD-LIRD model (2 mg of fluorescein i.p. followed 3 min later by a 4 min exposure to 54 Klux of white light; see I, J). Each symbol represents one eye (one eye per mouse was treated in each experiment). * ¼ p < 0.05, ** ¼ p < 0.01, *** ¼ p < 0.001.