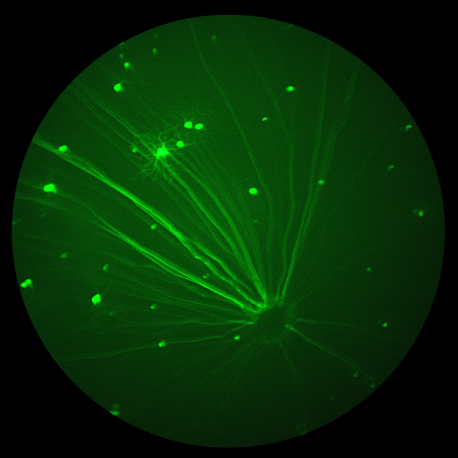
Diabetic retinopathy is a crippling complication of diabetes that can lead to loss of vision, characterized by retinal inflammation, neurodegeneration, and disorganized microvascularization . Oxidative stress is crucial to the development of diabetic retinopathy. Iron is an essential micronutrient but, in excess, can become a highly damaging oxidative species. Excessive iron has been implicated in Parkinson’s and Alzheimer’s diseases, age-related macular degeneration, cataracts, and glaucoma. Chaudhary et al sought to elucidate the connection between iron and diabetic retinopathy; they found that mice with excess iron fared worse with diabetic retinopathy than wildtype mice with the same disease. The excess iron-mice experienced increased cell death, vascular alterations—as seen with fundus images using the Phoenix MICRON® and fluorescein angiography–and loss of retinal barrier integrity.
Chaudhary et al used human homeostatic iron regulator protein (HFE) knockout mice; HFE regulates iron uptake and is expressed in the retinal pigment epithelium. HFE-knockout mice have increased iron levels. HFE-knockout mice experience retinal degeneration along with iron accumulation in the retina. Diabetes was induced in wildtype and HFE-knockout mice using streptozotocin. Interestingly, both diabetic mice and human retinas showed increased accumulation of iron. The blood-retinal-barrier is essential for maintaining retinal health; in diabetic HFE-knockout mice, it was much more disrupted than the WT mice. By culturing retinal pigment epithelium cells from HFE-knockout and wild type mice in glucose, Chaudhary et al directly linked retinal iron overload to the disruption of the blood-retinal-barrier.
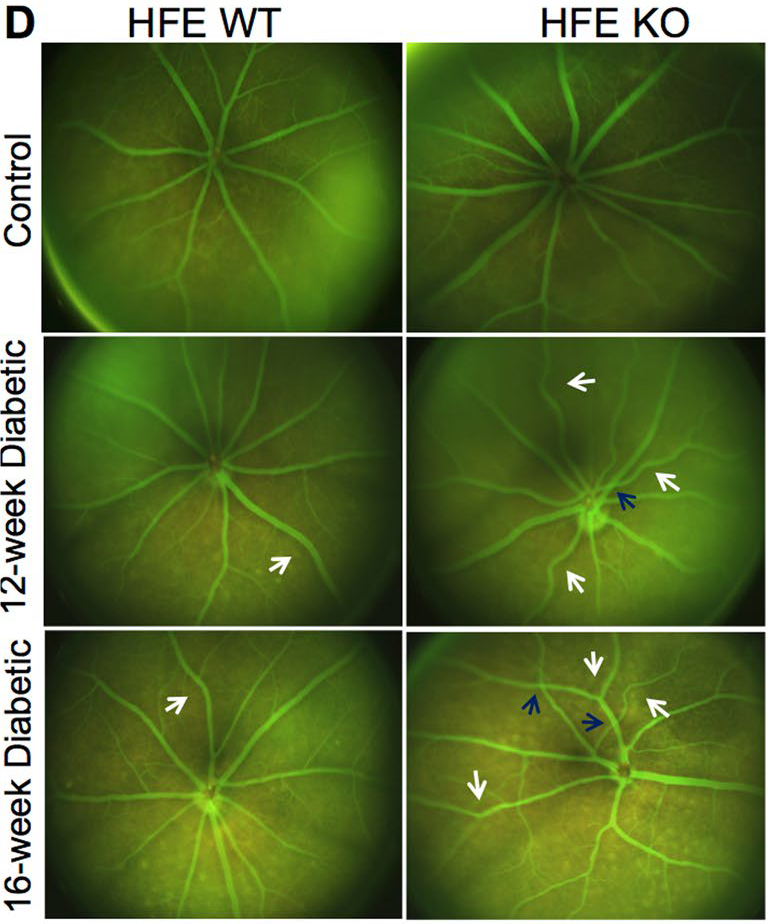
Using the Phoenix MICRON® fundus camera, the retinas of the mice were examined. The bright field and fluorescein angiography images of the non-diabetic HFE-knockout and wild type mice were normal whereas all diabetic mice showed an increase of pigment and vascular abnormalities. The vascular abnormalities appeared earlier in the diabetic HFE-knockout mice than in the diabetic wildtype mice. Furthermore, the vascular tortuosity, crossings, and retinal vein occlusions were much worse in the diabetic HFE-knockout mice than in the diabetic wildtype mice as the disease progressed (Fig 1).
In conclusion, Chaudhary et al provide detailed insight into the role of excess iron in diabetic retinopathy, from the protein level to fundus imaging with the Phoenix MICRON® camera. Detailed and crisp fluorescein angiography images show subtle and extreme changes in the vascular morphology, indicating that excess iron worsens the outcome of diabetic retinopathy and provides a potential therapeutic target.