Researchers at the University of Laval in Québec, Canada discovered unexpected findings with the Phoenix full field Ganzfeld electroretinography (ERG) system studying Alzheimer’s model mice. ERG assesses the function of the retinal cells including the photoreceptors, bipolar cells, and amacrine cells by flashing light at the retina and recording the electrical responses of the cells. By examining the height and speed of the electrical response wave forms, the retinal function integrity can be measured. The Phoenix Ganzfeld ERG system flashes green or UV light on the entire retina, which can tease out the function of rods, M-cones, and S-cones separately. The corneal electrode is the tip of an infrared light-sensitive camera which, along with an animal stand with many degrees of micro-manipulation, allows simple and precise alignment with the eye.
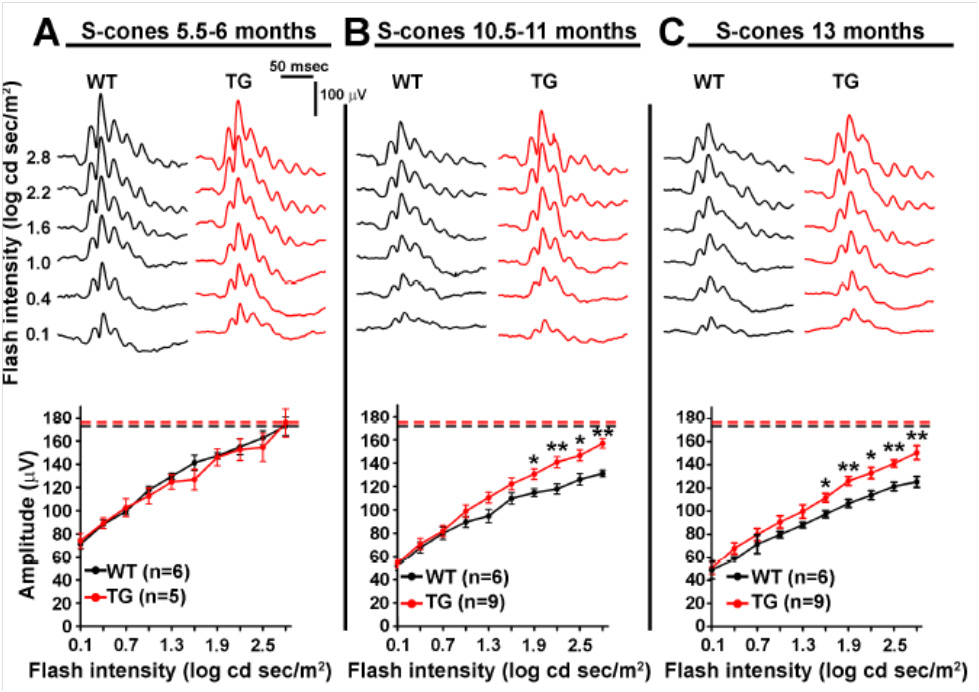
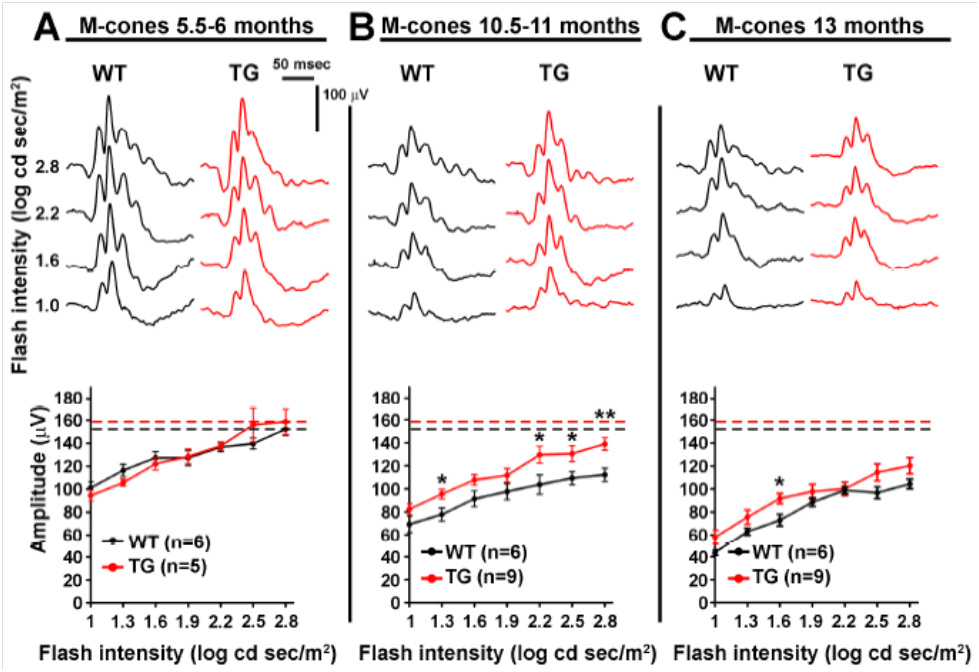
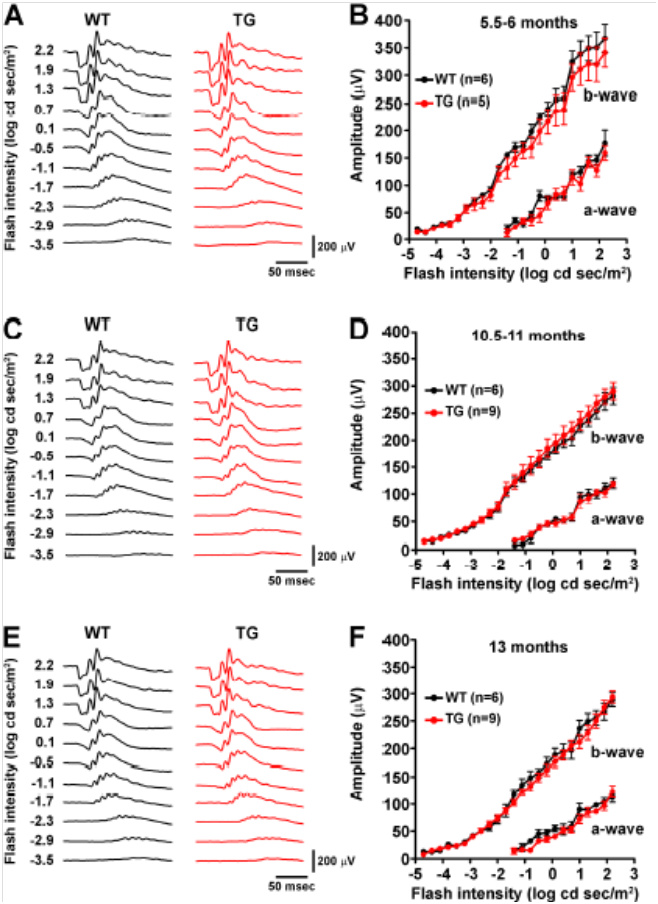
Joly et al used these separate measurements to examine the retinal function of wild type (WT) and Alzheimer’s model mice as they aged. Oddly, though the retinal function of both the Alzheimer’s and WT mice declined with age, the Alzheimer’s model mice preserved S-cone (Fig 1) and M-cone (Fig 2) function better than the WT mice. The rod function was similar between the Alzheimer’s and WT mice at all ages (Fig 3). They theorize that the retina and cone function may be protected from Alzheimer’s by way the retina processes amyloid.
Alzheimer’s disease is characterized by beta-amyloid plaques in the eyes and brains of elderly patients. In humans, Alzheimer’s is commonly linked with visual deficits such as decreased visual acuity. Joly et al examined APPswe/PS1∆E9 transgenic mice which are widely used to study the retina. The combination of human APPswe and PS1∆E9 expression causes accelerated amyloid deposits in the brain and induces learning and memory deficits. Some researchers have found beta-amyloid plaques in the retina while others have not, and ERG results were mixed—studying M- and S-cones separately had not been done. Joly et al performed scotopic and photopic ERG with green and UV light. All photoreceptor functions declined with age and the rod function was the same between WT and Alzheimer’s mice. However, the S-cones b-wave response declined less in Alzheimer’s mice compared to WT mice at 10.5-11 months and 13 months of age (Fig 1). Similarly, the M-cones b-waves were better in Alzheimer’s mice than WT mice at 10.5-11 months of age and trended toward improved at 13 months of age.
Joly et al discovered that while the brains of the aged Alzheimer’s mice contained beta-amyloid and plaques, the retinas of these mice did not. They theorized that amyloid precursor protein upregulation may be beneficial to photoreceptors and that there is a retina-specific processing of amyloid that enhances cell function in the aging retina.
Find this article in full with this citation: Joly, S., Lamoureux, S., Pernet, V., Non-amyloidogenic processing of amyloid beta precursor protein is associated with retinal function improvement in aging male APPswe/PS1ΔE9 mice, Neurobiology of Aging (2017), doi: 10.1016/j.neurobiolaging.2017.02.004.
Figure 1. UV flashes revealed age-related decline in S-cone function from 5 months (A) to 10 months (B) to 13 months (C). Surprisingly, Alzheimer’s mice S-cone b-wave amplitude declined less with age than the WT mice (B and C).
Figure 2. Photopic green flashes showed age-related decline in M-cone function from 5 months (A) to 10 months (B) to 13 months (C). Similarly to S-cones, Alzheimer’s mice M-cone b-wave amplitude declined less with age than the WT mice (B).
Figure 3. Scotopic green flashes showed no difference in rod function between Alzheimer’s and WT mice.